(when electrons are shared between nonmetals)
We learned how to draw Lewis Dot Diagrams (again)..
Don't know how? WELL I WILL TEACH YOU :)
Steps to drawing Lewis Dot Diagrams:
1) Add the the valence electrons in all atoms
2) Identify which atom can form the most number of bonds
- (the atom that has MORE potential to form bonds is called the CENTRAL atom)
3) Bonds between 2 atoms is shown by a line (---) which shows two electrons
4) Any electron that isn't paired, are paired with the remaining atoms
5) All valence shells must be filled and all electrons must be used (if not paired)
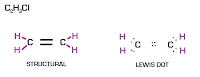
 DOUBLE & TRIPLE BONDS
DOUBLE & TRIPLE BONDS
(some compounds form more than one bond)
For example:
No comments:
Post a Comment